The molecular structure of forest aroma has been deciphered
For the first time, researchers accurately identified the chemical structure of alpha pinen – a compound formed by coniferous trees – in its gas phase. The new analysis could help scientists better understand how alpha pinen and possibly other similar molecules react with other gases in the atmosphere, as well as pollutants and particles emitted by aerosols that affect health and climate. The results of the study are published in the Journal of Chemical Physics by AIP Publishing.
“What you’re breathing in the forest is biogenic volatile organic molecules released into the atmosphere,” said Teresa Thuet, a physicist at the University of Lille in France. Determining the structure of these molecules, she said, “paves the way for future spectroscopic detection and simulation of atmospheric chemistry.”
Among the biogenic volatile organic molecules is a class of compounds called monoterpenes that react with ozone, hydrocillary radicals, nitrogen oxides and other gases in the atmosphere. These reactions create pollutants and aerosols that, for example, can condense more clouds and contribute to climate cooling. The main monoterpene is alpha-pinen, which annually in the lower layer of the atmosphere – the troposphere is produced by forests of about 50 trillion grams.
To accurately predict how alpha pine reacts in the troposphere, and therefore how it affects climate and air quality, researchers need a detailed understanding of its molecular structure. Alpha Pinen in its solid phase has already a certain bicyclic or two-ring structure. However, in the troposphere, alpha-pinen is in a gaseous state and noticeable changes in the structure have been detected due to the forces created by the crystalline structure.
Since alpha pinen exists in the gas phase only at low concentrations requiring highly sensitive experimental methods, no one has previously defined its gas-phase structure. “Until now, the definition of the structure of complex molecules, such as monoterpenes, is only possible in the condensed phase,” says Heuet.
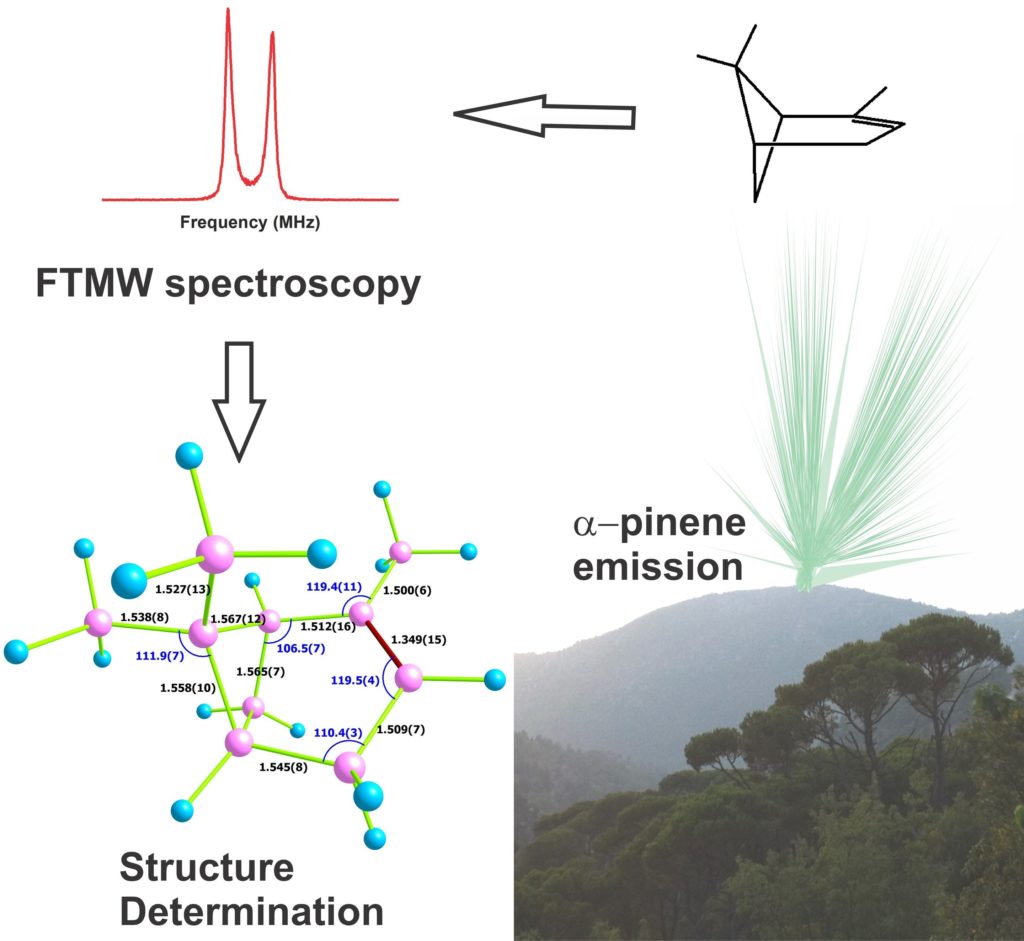
Experimentally presented gas-phase structure of alpha pinens using microwave spectroscopy with the transformation of Fourier and quantum chemical calculations
Huet’s lab has developed methods that are sensitive enough to determine the structure of monoterpene gases.
Researchers have identified quantum parameters called rotary constants that best describe data without structural assumptions. They repeated this analysis for all natural isotope versions of alpha pinens, in which carbon-13 isotopes replace different carbon-12 isotopes in the molecule. This set of rotary constants describes the full structure of alpha-pinen gas.
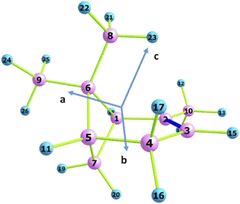
The molecular structure of α-pinens, calculated at the level of MP2 / aug-cc-pTV, and the numbering of all atoms. C and H atoms are shown blue and pink, respective
lySource:www.sciencedaily.com