Nanocomposite materials have been created for membranes to produce pure hydrogen
Russian scientists from the G.K. Catalysis Institute Boreskov of the Siberian branch of the Russian Academy of Sciences created nanocomposite materials for membranes that allow to receive pure hydrogen. The research is supported by a grant from the Russian Science Foundation (RPF). The scientists’ paper is published in the International Journal of Hydrogen Energy.
The demand for hydrogen fuel is growing every year, and we are projected to see a sharp increase in demand for hydrogen in the 21st century. This will be due to the increase in the depth of oil production, with the development of ammonia, methanol, liquid fuel, processes for obtaining quality iron and the development of hydrogen transport.
The easiest way to produce hydrogen is electrolysis – the process of passing electric current through the water solution of certain salts. The efficiency of this method is extremely low, so the promising method of obtaining hydrogen today is considered to be the conversion of the main component of natural gas – methane. Conversion is the process of turning some gases into others, occurring at high temperature. Thus, a mixture of methane and water is a mixture of carbon dioxide and hydrogen. Ethyl alcohol (ethanol) can also be used as the original fuel for hydrogen production. To increase the efficiency of this method of hydrogen production, it is necessary to use catalysts – special materials that accelerate the flow of the reaction.
Today, special membranes (elastic membranes) are used to effectively release hydrogen from the mix of reaction products. The most promising membranes are from dense materials. They allow hydrogen to be released from a mixture of gases formed after the process of conversion, but do not pass the molecules of the original substances (methane or ethanol) and by-products such as carbon monoxide and carbon dioxide. In a chemical reactor, a porous layer of catalyst is applied to the surface of the membranes in contact with the fuel mixture, in which the reactions of the steam conversion of biofuels (methane or ethanol) occur. Hydrogen from the mixture of products is transferred through the membrane to the other side, after which it can be identified and used.
Scientists from the G.K. Catalysis Institute Boreskova SO RAS developed a nanocomposite material consisting of neodim tungsten and nickel alloy nanoparticles with copper. It conducts hydrogen well through it and has high stability in working conditions. Scientists applied thin layers of this nanocomposite to a substrate of nickel-aluminium foam and then coated it with a porous layer of catalyst. This allowed the creation of catalytic membranes to produce pure hydrogen from biofuels.
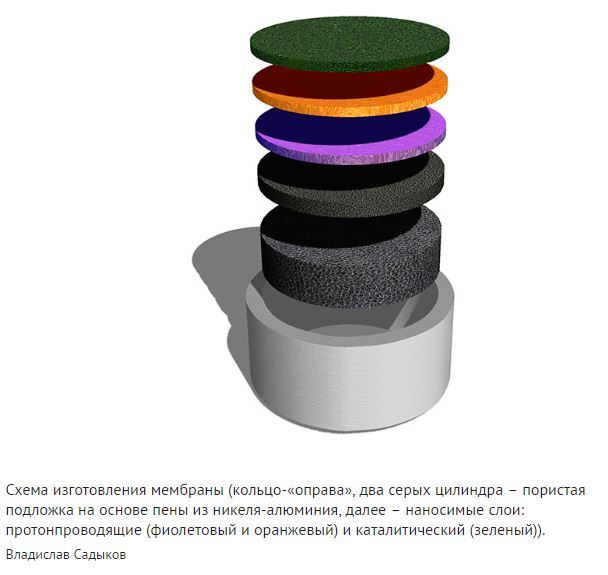
“Compared to the standard material for membranes – palladium – or its alloys, our nanocomposites are much cheaper and their effectiveness meets the requirements of practice,” says Dr. Chemical Sciences, head of the deep oxidation catalyst laboratory at the G.K. Institute of Catalysis. Vladislav Sadykov.
As part of the project, supported by the RPF grant, scientists have identified important physical and chemical characteristics of the materials received, including hydrogen permeability of membranes, working parameters of the processes of steam conversion of methane and ethanol in membrane reactors, the resource of work (the time during which the catalyst and membrane can function without degrading their properties). Measurements have shown that the materials obtained by the researchers allow effective conversion of fuels in membrane reactors with the release of pure hydrogen and have characteristics and a resource of work that corresponds to modern industrial requirements.
“The technology of the synthesis of our materials and the design of the membrane reactor have been worked out at the laboratory level. Going to the pilot level is the task of the near future. The introduction of catalytic membranes at the industrial level requires significantly more investments,” sums up Vladislav Sadyk
ov.Source:indicator.ru