The bonds between the layers of two-dimensional materials have proved mysteriously strong
sUniversity of Tsukuba
Physicists experimentally investigated the interactions between layers of matter consisting of flat atomic sheets. The results showed much stronger connections than the theory predicts. The paper describing the experiments is published in the journal Nature Materials.
The discovery of graphene, the first known flat material, with its distinctive exceptional properties, such as record-breaking strength to weight, flexibility, electronic conductivity and the ability to form impenetrable walls, has generated explosive interest in such substances. Many interesting, both fundamentally and appliedly, the quality of such systems are determined by the presence of long-range (compared to covalent or ion) connections. In physical chemistry, these types of interactions are described by van der Waals. One of their manifestations is to hold several layers of similar materials together.
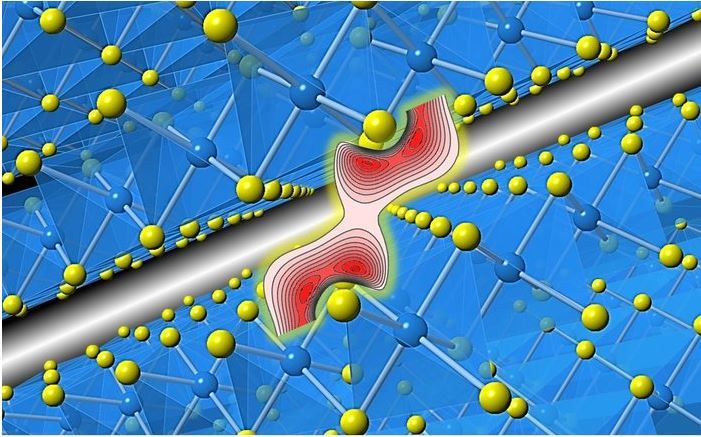
In a new paper, researchers at the University of Japan and the University of Aarhus in Denmark directly investigated the distribution of electronic density between layers of a typical two-dimensional material, the TiS2 titanium titanium disulfide. Within the layers of such matter, strong bonds are formed between titanium and sulfur, and between layers – weak long-range van der Vaals forces between sulfur atoms.
"The interaction between layers in van der Vaals materials such as TiS2 is a significant factor in the ability to modify, process, and assemble them," explains co-author Eiji Nishibori. "By modeling the experimental results from synchrotron and comparing them with calculations within the theory of density functionality, we got amazing information about the nature of electronic exchange between layers."
As a result, it turned out that there is a significant exchange of electrons between the layers, and the connections themselves are much stronger than the theory predicts. The authors note that the excellent agreement of the results with the data on interactions within the layers only confirms the validity of the found discrepancy in the description of the impact in the inter-layered interval. Scientists hope their work will provide a better understanding of the nature of weak connections, which may prove important in areas such as ion batteries, superconductors and catalyst creatio
n.Source indicator.ru