Porous protein crystals will store molecules inside cells
Japanese bioengineers have developed protein crystals with a porous structure capable of holding exogenous molecules inside living cells. In the future, such porous crystals can be used, for example, for intracellular delivery of drugs or enzymes. The article is published in the journal ACS Nano.
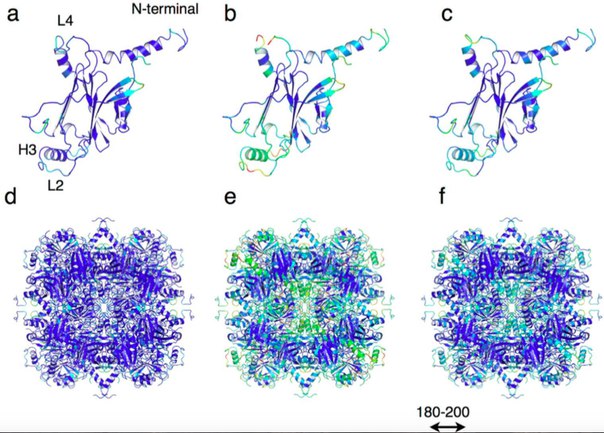
The prototype for protein crystals was the protein shell of cipoviruses, which cause polyedrosa in insects. These viruses are enclosed in multifaceted protective protein crystals called polyedrami and have very high stability. This stability is provided by the dense packaging of polyedrine monomers in trimers, which form a crystal with low porosity, limiting the inclusion of foreign molecules.
The authors hypothesized that increasing the porosity of polyedrine crystal while maintaining its stability would allow it to be used to capture and store exogenous molecules in living cells. To increase porosity, scientists used genetic engineering techniques to remove amino acid residues on the contact surface of each trimer. This allowed to preserve the original structure of the crystal lattice, but at the same time to increase the size of the pores.
Scientists have also tracked how mutant crystals behave inside living insect cells. The crystals were very stable and able to accumulate paint directly inside the cells.
The study opens up prospects for the development of nanomaterials with adjustable porosity from natural self-organizing protein crystals. Such porous crystals can be used, for example, for intracellular delivery of drugs or enzymes. Also, protein pores can be used for structural analysis of cell molecules by crystallization method.
Crystallization is one of the main methods of studying the structure of membrane proteins. Large crystals of proteins are studied using X-ray crystal imaging techniques, which allow to determine their molecular structure very accurately from the pattern of radiation scattering. Recently, another group of Japanese scientists described the crystallization of light-sensitive membrane proteins of bacteriorodopsins. It turned out that during crystallization they behave like "cannibals": the growth of large crystals is due to the "eating" of smaller crystals around them.Source https://texnomaniya.ru